About the ADDRESS-LC
Long COVID Study
What Does Participation Involve?
The ADDRESS-LC Long COVID study is designed to recognize the challenges of living with Long COVID. Participants will attend 5 visits at the doctor’s office plus 1 virtual visit from home over 5 months. The visits at the doctor’s office will each last about 2 to 3 hours. Accommodation for cognitive exertion will be provided at each visit as needed.

The first visit will include:
- A detailed review of the study and what is expected to participate.
- If you choose to participate, you will sign an agreement (informed consent form) confirming your desire to participate.
- The study staff will confirm your medical history, including your history of Long COVID. A physical exam and blood tests will be performed.
- Study assessments, including assessments of cognitive function and fatigue, will be conducted.
- Once it is confirmed you qualify to participate, you will return for Visit 2 within 2 to 4 weeks.
At Visit 2, you will:
- Complete study assessments focused on your symptoms of Long COVID
- Begin the study medication.
You may receive either the active investigational medication, bezisterim, or placebo capsules to be taken two times a day. Neither you, the study physician nor any study staff will know whether you are receiving the active medication or placebo in order to demonstrate whether bezisterim can improve symptoms of Long COVID better than placebo.
Visits 3 through 5 will be similar to Visit 2
Once you complete the study medication and assessments at Visit 5, you will have a virtual telemedicine visit for the final visit, Visit 6, from your home.
Everything needed to participate is provided without cost and compensation may be available for participating.
Overview of study visits
- 5 visits at the doctor’s office plus 1 virtual visit from home
- Visit 1 to confirm initial eligibility followed by 5 visitis over 16 weeks
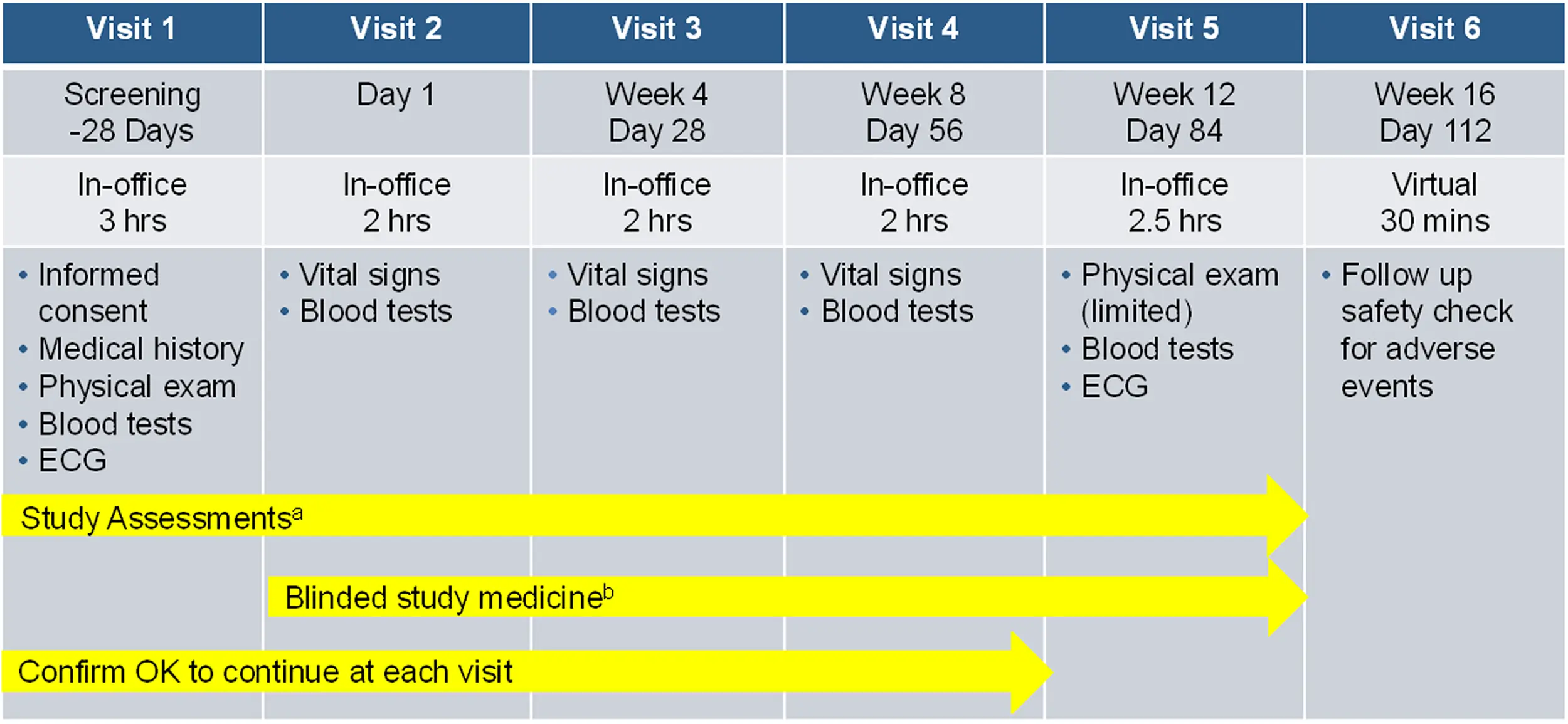
a. Clinician assessments and participant questionnaires to assess symptoms of Long COVID.
b. Take one capsule of blinded study medication 2 times daily (morning/evening).
Potential Side Effects
In completed clinical studies of bezisterim, when bezisterim was compared to placebo, the most commonly observed side effect with bezisterim has been headache which occurred at rates similar to placebo. Other possible side effects have not been seen more frequently with bezisterim treatment compared to placebo. These effects may include increases in blood glucose levels or cholesterol levels and decreases in blood calcium levels or blood sodium levels. This is not a full listing of all possible observed side events. For a full list of side effects, talk with the study physician or reference the study documentation (informed consent) when you enroll.
Is This Study Right For You?
The ADDRESS-LC Long COVID clinical study may not be what you’re looking for. Half of the people in the study will get placebo (non-active, like a sugar pill) instead of the investigational drug bezisterim, and there’s no way to know what you are going to get. If you decide that you want to try other medicines for Long COVID while on the study, you will have to stop being in the study.
